- Aguettant Nordic
- AGUETTANT nordic
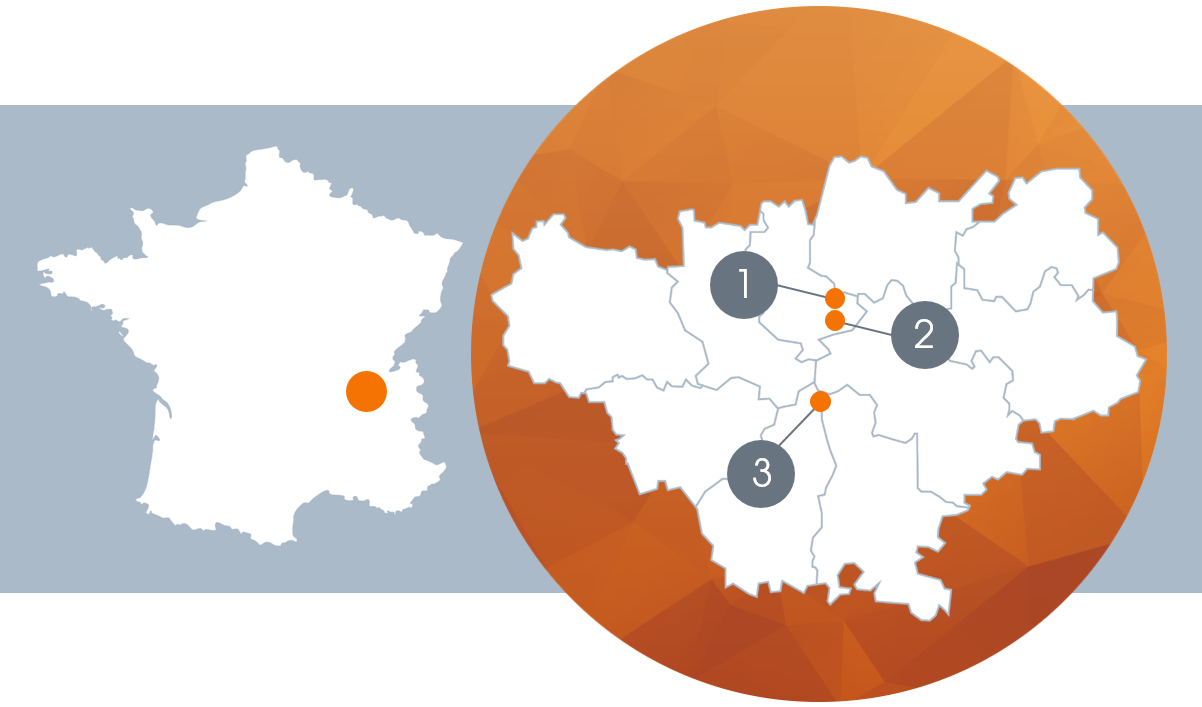
- Our establishments in France
Our establishments in France
Optimised processes and technological innovation are leading to high-performance production sites. The in-house production cycle operates at the highest standard to maintain consistent production of quality products. Laboratoire Aguettant is operating two production plants, one logistics platform and R&D laboratories, all located in France.
1. LYON-GERLAND
GMP (ANSM) site, ISO 13485-certified (GMED). Production of high quality medicines: everything from formulation, filling and packaging is manufatured in a controlled zone.
2. SAINT-FONS
GDP (ANSM) site, ISO 13485-certified (GMED). A fully integrated logistics platform and customer services, from the first confirmation of the purchase order to the delivery of the goods.
3. CHAMPAGNE
GMP (ANSM) site, ISO 13485-certified (GMED). High volume production of sterile products in plastic from 5ml to 10 litres (blow-fill-seal technology).
Our extensive quality management system is designed to assure the quality of our products. Every year, Aguettant manufacturing sites produce more than100 MILLION MEDICINE UNITS delivered in more than 70 countries.
